Formic Acid Uses, Specifications & Technical Data Sheets
Overview
Application
Specification
Overview

Some of the top players in formic acid are BASF, Eastman chemical company, and Feicheng acid chemicals. When studied geographically Europe, especially Germany and Mainland China are the top suppliers for formic acid in the world.
The Manufacturing Process
- With methyl formate and formamide – When methanol reacts with water, methyl formate is formed. This on further hydrolysis gives formic acid.
CH3OH + CO → HCO2CH3
HCO2CH3 + H2O → HCO2H + CH3OH
- Ammonia can be used. Methyl formate reacts with ammonia to form formamide, which on treatment with sulphuric acid gives formic acid.
HCO2CH3 + NH3 → HC(O)NH2 + CH3OH
2 HC(O)NH2 + 2H2O + H2SO4 → 2HCO2H + (NH4)2SO4
Many manufacturers use the first route to avoid the formation of by-products formed in the second route.
- In the laboratory, formic acid can be obtained by heating oxalic acid in glycerol. Glycerol acts as a catalyst, as the reaction proceeds through a glyceryl oxalate intermediary.
C2O4H2 → CO2H2 + CO
Application
Formic Acid Applications
- Food and Beverage : Having an E number from European Food Safety Authority (EFSA), formic acid is classified as a preservative in the food industry with an E number of E236.
- Agriculture : formic acid uses in agriculture industry is working as an active ingredient of pesticide to directly kill the mites around beehives without disrupting the bees or the life span.
- Textile : Similar to the leather industry, textile industry used formic acid to neutralize excess alkaline in textile manufacture.
- Leather : Leather industry used formic acid to homogenize the tanning of hides and penetrate collagen fibers. Formic acid neutralize the excess alkali involved in a tanning processing.
- Other Applications: Formic acid is used instead of mineral acids in some cleaning products such as limescale remover and toilet cleaners. Its esters are used in the making of perfumes. It is also used as a coagulant in rubber production. Also formic acid uses in medicine is reported to be an effective treatment for warts.
Specification
FORMIC ACID SPECIFICATIONS
| CAS NO | 64-18-6 |
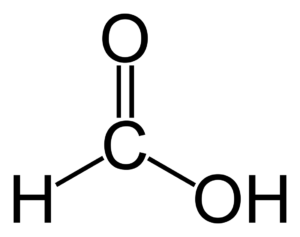
| FORMULA | HCOOH |
| H.S CODE | 29151100 |
| MOL WT | 46.025 g mol-1 |
| SYNONYMS | Methanoic acid, Formylic acid, Hydrogen carboxylic acid |
PHYSICAL AND CHEMICAL PROPERTIES
| PHYSICAL STATE | Clear liquid |
| MELTING POINT | 8oC |
| BOILING POINT | 101oC @ 760 mmHg |
| SOLUBILITY IN WATER | Miscible. |
| PH | 3.47 (1mM) |
| SPECIFIC GRAVITY | 1.220 |
| NFPA RATINGS | Health: 3; Flammability: 2; Instability: 1 |
| STABILITY | Hygroscopic: absorbs moisture or water from the air. Keep Refrigerated. Formic acid may decompose to carbon Monoxide and water or carbon dioxide and hydrogen gas. These decomposition products develop pressure. Heat Sensitive. |
